Global
pharmaceuticals
for Japanese
patients

ABOUT US
Huckleberry is a Japan-based management company for Health Care Professionals, 100% owned by Outrigger Investments, founded 2020. We also provides importing medicines and medical devices procedures instead of the Health Care Professionals and obtain new drugs approval.
Our business is to import new medicines from overseas at the request of physicians, taking advantage of our strong connections with university hospitals through joint projects. Once new medicines are adopted by university hospitals, small and medium-sized hospitals, aesthetic clinics, and dental clinics, other buyers will generally follow. We negotiate with overseas pharmaceutical companies, establish import routes, and complete the import certification procedures with the Japanese Ministry of Health, Labor and Welfare. Huckleberry runs an important role by supplying Japanese patients with the opportunity to receive medical care through new medicines that are not available in Japan.
-
PROMOTE
EFFECTIVE
MEDICINES
TO JAPAN
-
CONTINUE
TO PROVIDE
MEDICATIONS
TO PATIENTS
-
OBTAIN
NEW DRUGS
APPROVAL
SERVICE
Importing medicines to Japan is subject to control by the Pharmaceutical Affairs Law and the Customs Law to prevent health hazards caused by defective products. Although health care professionals are only allowed to import medicines at their own responsibility, they don’t
research medicines or prepare the required legal paperwork themselves. Due to the
specialization of health care professionals, Huckleberry researches effective medicines from overseas and processes the required legal paperworks to obtain the required medical import certificates.
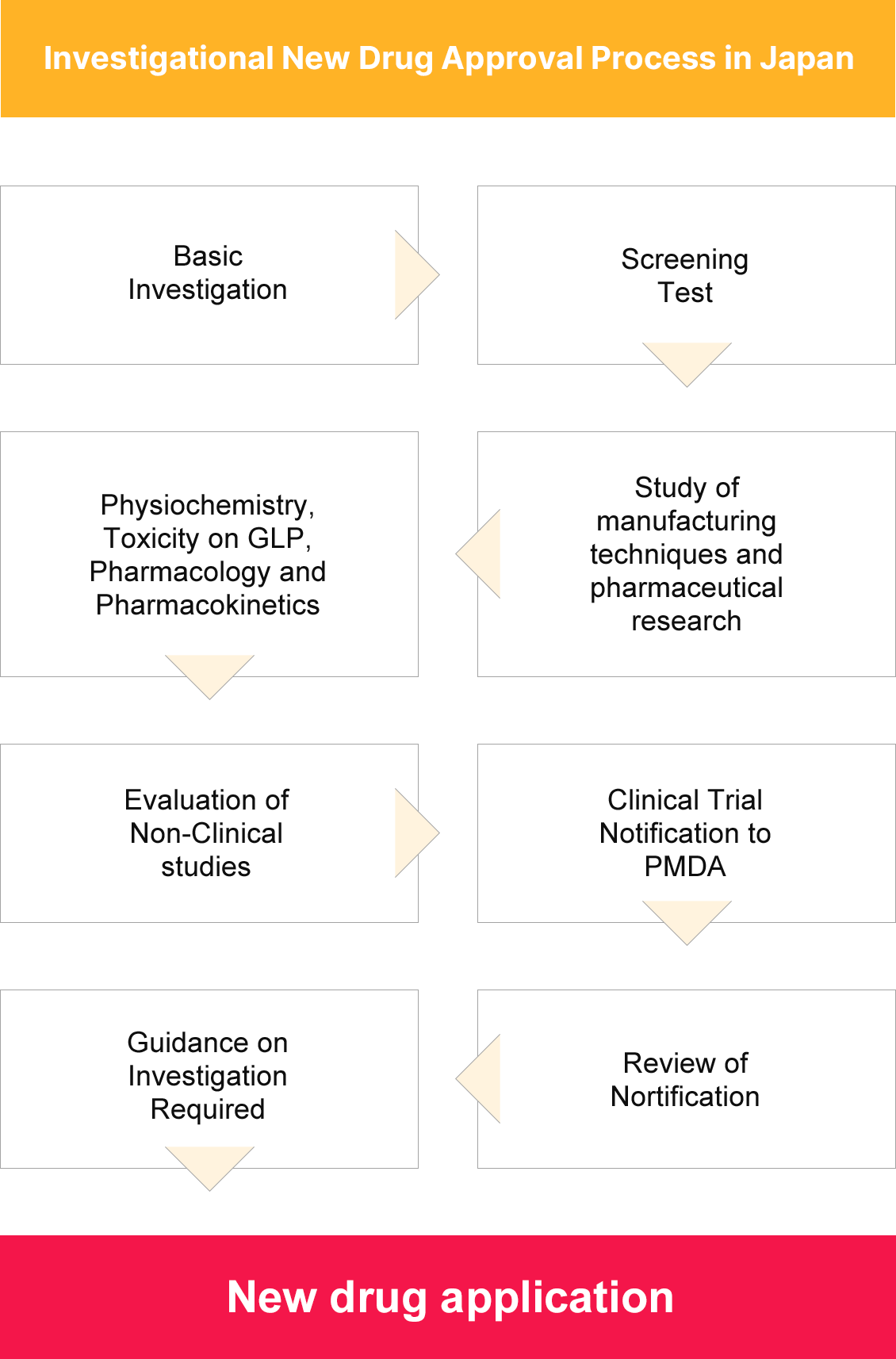
Strategy to Obtain Medical Import Certificates in Japan
Non-registered case


CAREERS
Our goal is to become a company that promotes effective medicines from overseas to the Japanese market. Firstly, we work with health care professionals to import the medicines with their own responsibility according to Strategy to Obtain Medical Import Certificates in Japan. Secondly, we
proceed to take registrations for the new medicines. In order to register new drugs in Japan, in addition to our internal management team, we are working on several projects with partners who have extensive background such as his drug investigation experiences at US pharmaceutical companies. Due to work with them, some of the critical points will be clear before registering the drugs.

Japanese Pharmaceutical Market
In 2021, the Japanese pharmaceutical market was the second largest in the world at $85.4 billion. The Japanese pharmaceutical market tends to
generally follow the medicines that university hospitals adopt. Once adopted by a university hospital, there are tendencies for other organizations such as public hospitals, small and medium-sized hospitals, aesthetic clinics, and dental clinics, to follow the same medicines. In the case of importing non-registered drugs from overseas to Japan, health care professionals (*1) must obtain permission from the Ministry of Health, Labor and Welfare (*2) through their own license. In other words, they are only allowed to import medicines for patients at their own responsibility when required legal procedures done. Thus, non-registered drugs from overseas are in high demand in the Japanese market.
- *1 in this case, said licensed physicians and dentists
- *2 a cabinet level ministry of the Japanese government
COMPANY
Management Team
Ryo Narui
CEO
Ryo began his finance and business development career over 18 years ago. In 2003, Ryo was heavily involved with an investment company where he was responsible for market analysis and mergers & acquisitions in central Tokyo. In 2007, he became an asset manager at Morgan Stanley Japan for global real estate funds and successfully invested $890 million of capital into assets and managed $1.3 billion in assets. He previously founded and has been actively involved in several start-up ventures over the past decade in consumer products and medical industries. Ryo is also the CEO of Outrigger Investments, a real estate and business investments firm.
Hiroshi Watanuki
Sales&Business Development
Hiroshi began his career in the pharmaceutical industry in 2005. He joined The US-baced skin care company as a senior manager of sales planning in 2013. He has been involved in drug development proposals, negotiations with the Ministry of Health, Labor and Welfare, and cadaver (autopsy facility) negotiations with university hospitals.
Toshi Inoue
Sales & Marketing
Toshi began his career in the health care industry. He led the product development team responsible for sourcing, designing and developing new products, as a product planner. In 2017, he joined the Japan branch of France-based
cosmetics company as the project manager in the professional aesthetic products division. He was involved in planning and development, and market research for aesthetic clinics. He currently runs EC aesthetic stores and investment businesses.
